BOFAP, tested antimicrobial effectiviness
Bofap reduces up to 99.9% of particulate matter with a size of 0.2 microns
Cross-contamination during spirometer use can be of two types:
– By epidermal contact, which can be resolved by simple disinfection
– Inhalation of pathogens placed in the instrument by previous patients during exhalation inside the spirometer
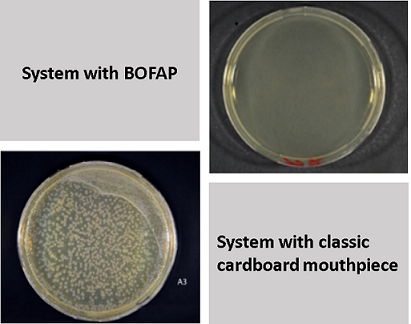
One of the peculiarities of BoFAP is that it drastically limits the risk of cross-contamination between patients and between patient-operator, thanks to the barrier action exerted by the built-in filter, which is able to block most particulate matter. The effectiveness of a filter must be verified as the percentage abatement capacity of a known microbial load passed through the filter. In addition, the single packaging method adopted by LUMED has proven to be instrumental in the microbiological protection of consumable material for spirometry use. In fact, with such a packaging method, the surface microbial load is practically eliminated.
With the collaboration of several competent facilities, LUMED srl has verified the microbial barrier effectiveness of BoFAP
TESTED ANTIMICROBIAL EFFECTIVENESS
To test the antimicrobial efficacy, the barrier capacity of filter systems was evaluated using a microbial aerosol nebulised in a system capable of generating a flow of air comparable to that of the patient’s exhalation phase. As the image below shows, the flow generated for the simulation passes first through the mouthpiece then through the filter on which the Petri dish loaded with TSA, (Triptic Soy Agar) is placed. The result clearly shows the difference in the development of viable bacterial and viral cells on the Petri dish; in fact, far fewer colony-forming units are present on the dish mounted on the system with the BoFaP than on the dish mounted on the system with the classical mouthpiece